Nitrate in the Upper Republican Natural Resources District’s Groundwater
Nitrate in the Upper Republican Natural Resources District’s Groundwater
The Upper Republican Natural Resources District’s (URNRD) water quality sampling program began in 1974 by testing for nitrate in 18 wells. Over the years, the District has modified and expanded the sampling program so that in 2020 the District was sampling and testing 503 wells for nitrate. The goals of the nitrate sampling are twofold – identify and reduce human health risk and improve the general health of the aquifer beneath the URNRD - and to fulfill these goals, the URNRD preforms water sampling and testing in both the summer and winter.
Summer sampling focuses on the general aquifer health within the District. The URNRD samples 144 irrigation wells every year to estimate the average nitrate concentration throughout the district. While most of the District has relatively good water quality, some areas have higher nitrate concentrations, and in those areas the URNRD requires landowners and operators to follow farm management practices that will maintain or reduce nitrate concentrations in the groundwater.
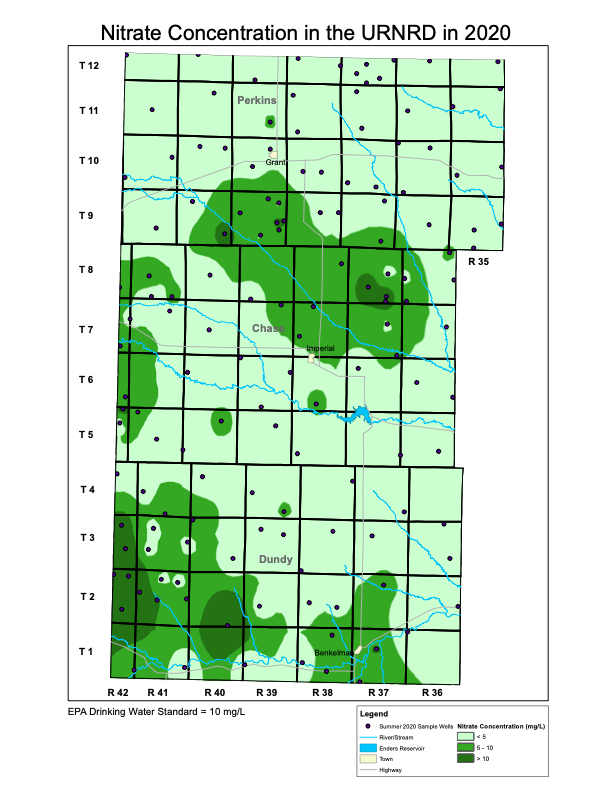
The figure titled Nitrate Concentration in the URNRD in 2020 shows the results of the summer testing and the locations of the sampled wells. Most of the groundwater in the District appears to have nitrate concentrations below the U.S. Environmental Protection Agency’s (EPA) drinking water standard for nitrate, although there are areas in southwest Dundy County and northern Chase County/southern Perkins County that exceed the nitrate drinking water standard. In general, high nitrate concentration levels tend to occur in the Sand Hills regions of the District.
The winter sampling focuses on human health. There are 359 registered domestic wells in the District, and the District samples approximately 72 wells each year on a 5-year cycle, so all registered domestic wells are sampled every 5 years. If a well has a nitrate-nitrogen concentration greater than 10 mg/l, an URNRD representative will inspect the well’s construction information and site to determine the potential source of the high nitrate and provide potential solutions to solve their problems.
Source of Nitrate in Groundwater
Nitrogen is one of the primary nutrients necessary for plant growth, and the most common source of nitrogen used by plants is the nitrate ion, which is a combination of nitrogen and oxygen. Most nitrogen compounds are converted to nitrate, so all sources of nitrogen (i.e. organic matter, nitrite, ammonium, and nitrogen gas) should be considered as potential nitrate sources. Bacteria in the soil create nitrate from the other nitrogen compounds.
Nitrate is highly soluble and water entering the soil from precipitation or irrigation will carry nitrate, not used by plants, through the unsaturated soil and down to the aquifer. Nitrate may enter the groundwater from non-point sources (sources that contribute nitrate over a broad area), such as fertilized cropland, parks, golf courses, lawns, and gardens; from point sources (sources that introduce nitrate at a single location), such as feedlots, septic systems, chemical spills, leaking chemical storage facilities, and improperly abandoned wells; or from naturally occurring sources of nitrogen. High nitrate concentrations in the groundwater are usually a result of human influence.
Infiltrating water containing nitrate increases the nitrate concentration in the groundwater over time. Nitrate enters the top of the aquifer and slowly mixes with water deeper in the aquifer, so nitrate concentrations at the top of the aquifer are frequently greater than concentrations at greater depths. Once nitrate enters the aquifer, it essentially stays there. Nitrate removed from the aquifer in pumped water has little to no effect on the nitrate concentration in the groundwater. In general, the nitrate concentration in the District has gradually increased at about 0.04 mg/l per year.
Indications of Nitrate in Water
Because they cannot detect nitrate in drinking water by taste, sight, or smell, sampling and testing are the only way to determine the concentration of nitrate in water. A laboratory approved for nitrate testing should perform the test to determine the presence of nitrate in drinking water. The Nebraska Department of Health and Human Services (DHHS) approves laboratories to conduct tests for drinking water supplies. This approval means that recognized, standard test and quality control procedures are used. See Drinking Water: Approved Water Testing Laboratories in Nebraska (G1614) for a list of approved laboratories and contact information for each.
Potential Health Effects
The EPA Maximum Contaminant Level (MCL) for nitrate-nitrogen in a public water supply is 10 milligrams per liter (mg/L) and is based on acute health effects, specifically the risk of methemoglobinemia. Acute health effects are those that result from ingestion of a contaminant over a short period of time. The acute health hazard associated with nitrate in drinking water occurs when bacteria in the digestive system transform nitrate to nitrite. The nitrite reacts with iron in the hemoglobin of red blood cells to form methemoglobin, which lacks the oxygen-carrying ability of hemoglobin. This creates the condition known as methemoglobinemia (sometimes referred to as “blue baby syndrome”), in which blood lacks the ability to carry sufficient oxygen to the individual body cells.
Infants under one year of age have the highest risk of developing methemoglobinemia. Contributing risk factors include digestive and enzyme systems that are not fully developed. Older persons who have a gastrointestinal system disorder resulting in increased bacteria growth may be at greater risk than the general population. In addition, individuals who have a genetically impaired enzyme system for metabolizing methemoglobin may be at greater risk. When the nitrate-contaminating source is removed, the effects are reversible. The general population has a low risk of developing methemoglobinemia, even when ingesting relatively high levels of nitrate/nitrite.
Exposure to higher levels of nitrates or nitrites has been associated with increased incidence of cancer in adults. Although the EPA set the drinking water standard at 10 mg/L based on acute health effects, questions have been raised regarding possible chronic health effects from consuming water with nitrate at higher concentrations. Chronic health effects are those that can occur when a contaminant has been ingested over long periods of time. Research is limited regarding the possibility of chronic health effects due to long-term ingestion of drinking water with nitrate above the MCL. While it is recognized that research is limited, largely due to the complexity and cost of this type of research, some studies have shown a correlation between long-term ingestion of elevated nitrate and increased incidence of certain
cancers and birth defects. Uncertainty exists in nitrate risk assessment, and the connections between the level of nitrate in drinking water, volume ingested, duration of exposure, and possible chronic risks are not fully understood. The EPA concluded that there was conflicting evidence in the literature whether exposures to nitrate or nitrites are associated with cancer in adults and in children.
High nitrate-nitrogen concentrations can also cause methemoglobinemia in livestock, although livestock are less susceptible to methemoglobinemia than humans. Water with a nitrate-nitrogen concentration in excess of 100 mg/l is considered potentially harmful to livestock, although this limit also depends on the quantity of nitrate in feed and the age of the animal.
EPA Drinking Water Standard for Nitrate
The EPA, under the authority of the Safe Drinking Water Act (SDWA), has set the Maximum Contaminant Level Goal (MCLG) for nitrate-nitrogen at 10.0 mg/L. This is the health-based goal at which no known or anticipated adverse effects on human health occur and for which an adequate margin of safety exists. The EPA has set these levels of protection based on the best available science to prevent potential health problems. Based on the MCLG, EPA set an enforceable regulation for total nitrate, the Maximum Contaminant Level (MCL), at 10 mg/L (10 ppm). The EPA sets MCLs as close to the MCLG as possible, considering cost, benefits and the ability of public water systems to detect and remove contaminants using suitable treatment technologies.
While EPA and Nebraska regulations do not apply to private drinking water wells, private drinking water users may consider the EPA guideline of 10 mg/l nitrate-nitrogen in considering the risk associated with their water supply and try to reduce the nitrate-nitrogen concentration in the water, considering health risks, costs, and benefits.
Water Treatment Methods
Options for Public Water Supplies
The EPA and the Nebraska DHHS regulates the quality of water provided by public water supplies. Community water systems (CWS) serve at least 15 service connections or 25 residents year-round. They include such entities as municipalities, mobile home parks, rural water districts, and sanitary improvement districts.
Non-treatment alternatives that provide drinking water that meets the EPA standard for nitrate-nitrogen standard (10 mg/l) may be as simple as blending high nitrate concentration water with water having a low nitrate concentration, so the average concentration is below the EPA standard or drilling a new well where the nitrate concentration is below the EPA standard. In some cases, users may achieve compliance by offering bottled water to consumers in conjunction with developing a source water protection plan designed to eliminate or reduce the source of contamination in the well, which should result in the reduction of the nitrate concentration in the water supply over time.
Conventional processes such as ion exchange, reverse osmosis, and electrodialysis are widely used for nitrate treatment of drinking water for CWS. These processes physically or chemically remove nitrate
from drinking water. Because nitrate is a stable and highly soluble ion with a low potential for precipitation or adsorption, removal by processes such as filtration or activated carbon adsorption are not used.
Techniques that remove nitrates may have varying effectiveness based on the amount of nitrate in the water supply and the balance of other ions in the water. The ion exchange process, for example, is sensitive to waters containing high TDS, high sulfate, and high hardness levels (which can cause hardness precipitation during regeneration). Effective anion exchange removal of inorganic nitrate requires softening pretreatment ahead of the anion exchanger. Using nitrate “selective” resins is also recommended.
Reverse osmosis (RO) is a physical process in which contaminants are removed by applying pressure to force raw water through a semi-permeable membrane, allowing water to pass through while retaining dissolved minerals. In low pressure (<100 psi) applications, the amount of drinking water produced is only 10% to 25% of the raw water used. High pressure systems can achieve water efficiencies of greater than 85%, but require specialized pumps and significant energy to achieve this level of efficiency. Reverse osmosis is one of the most expensive forms of centralized treatment and will probably not be cost effective unless there are multiple contaminants needing removal.
In the electrodialysis (ED) process, a positive electrode (cathode) and a negative electrode (anode) charge an ion-selective semipermeable membrane, which attracts and removes oppositely charged ions, such as the nitrate ion, as the raw water passes through the membrane.
Options for Private Water Supplies
Non-treatment options for obtaining acceptable drinking water for private users include drilling a new well in a different location, especially if the nitrate contamination is from a point source such as livestock or human wastes, and purchasing bottled water.
Nitrate treatment systems for private water supplies come in Point-of-Entry (POE) and Point-of-Use (POU) systems. POE systems treat water before it enters the home, so all the home’s water will be treated. POU systems treat water where it is used. For instance, a POU system could be hooked up under the kitchen sink and only treat the water that comes out of that faucet.
Treatment options for reducing nitrate in private drinking water supplies include distillation, reverse osmosis, and ion exchange. Of these methods, reverse osmosis and ion exchange are the most common methods used for residences. Home treatment equipment using these processes are available from several manufacturers. Carbon filters and standard water softeners do not remove nitrate, and just boiling water will increase rather than decrease the nitrate concentration.
The distillation process involves heating the water to boiling and then collecting and condensing the steam using a coil. This process can remove nearly 100 percent of the nitrate-nitrogen. For information on this treatment method see NebGuide 1493, Drinking Water Treatment: Distillation.
In the reverse osmosis process, pressure is applied to water to force it through a semipermeable membrane. As the water passes through, the membrane filters out most of the impurities. This process can remove approximately 85 percent to 95 percent of the nitrate-nitrogen. Actual removal rates may
vary, depending on the initial quality of the water, the system pressure, membrane technology, and water temperature. For information on this treatment method see NebGuide 1490, Drinking Water Treatment: Reverse Osmosis.
Ion exchange for nitrate-nitrogen removal operates on the same principle as a household water softener. However, for the nitrate-nitrogen removal process, special anion exchange resins are used that exchange chloride ions for nitrate and sulfate ions in the water as it passes through the resin. Since most anion exchange resins have a higher selectivity for sulfate than nitrate, the level of sulfate in the water is an important factor in the efficiency of an ion exchange system for removing nitrate-nitrogen. For information on this treatment method see NebGuide 1491, Drinking Water Treatment: Ion Exchange.
The treatment methods listed above are recognized to be effective in reducing the nitrate concentration sufficiently to meet or exceed drinking water standards; however, this list does not reflect the fact that POU/POE devices and systems currently on the market may vary in their effectiveness in treating specific contaminants and performance may vary from application to application. The treatment system or combination of systems that will be best for a private well user depends on several factors including the nitrate concentration in the water, desired level of nitrate removal from the water, the quantity of water to be treated, and the chemistry of the water. Because the water chemistry may affect these treatment technologies, a complete water analysis should be conducted prior to any treatment application.
Generally, it is recommended that devices and systems that are independently certified to appropriate NSF/ANSI standards should be used. Whenever possible, assistance from a water professional or expert should be sought in the selection, installation, and operation of a chosen technique. Visit WQA.org to locate water professionals in your area. Note that Certified Water Specialists have passed the water treatment education program with the Water Quality Association and continue their education with recertification every 3 years.
Farm Management Recommendations and Requirements
The URNRD has created farm management recommendations and requirements to reduce or prevent the increase of nitrate concentration in the District’s groundwater. The URNRD is divided into four areas, or Phase Designations, that are based on the nitrate concentration and on whether the nitrate concentration is increasing or decreasing. Each Phase Designation has a unique set of farm management recommendations and/or requirements associated with it. For a more detailed discussion of the Phase Designation Areas and their farm management requirements, please see the section on the URNRD webpage pertaining to it (https://www.urnrd.org/farm-management-recommendations-and-requirements#overlay-context=programs-regulations).